Fullest Cerebrolysin Review 2023: Composition, Effects, Alternatives and How to inject?
June 1, 2023
Cerebrolysin Review Content
- What is Cerebrolysin?
- Cerebrolysin drug study and history
- Cerebrolysin intended uses
- Cerebrolysin composition: peptides, amino acides, vitamins, trace elements
- Cerebrolysin mechanism of action
- Is Cerebrolysin safe?
- Can Cerebrolysin be used in children?
- What are Cerebrolysin side effects?
- Cerebrolysin drug interactions
- Can Cerebrolysin cause prion disease?
- What is Cerebrolysin dosage?
- How to inject Cerebrolysin?
- Can i store an open vial of cerebrolysin?
- Can i split the contents of the vial?
- How to make an IV infusion?
- How to make a Cerebrolysin nasal spray?
- How to make a Cerebrolysin oral solution?
- What are Cerebrolysin Alternatives?
- Ever Pharma, Cerebrolysin Producer
- Is my Cerebrolysin authentic? CoA?
- Is it ok that the seal on the Cerebrolysin box is open?
- Where can I buy Cerebrolysin?
- Cerebrolysin Customer reviews
- Conclusion
- Bibliography
WHAT IS CEREBROLYSIN?
Cerebrolysin is a neurometabolic stimulator and nootropic peptide. It is based on low molecular weight biologically active neuropeptides (20%) and amino acids (80%) isolated from porcine brain tissue by enzymatic cleavage.
Cerebrolysin mechanism of action is all about the ability to activate endogenous defense mechanisms which include three main components: neuroprotection, neuroplasticity, and neurogenesis. Unlike the majority of peptide drugs, Cerebrolysin has all the amino acids which compose the central nervous system; this can explain many positive properties of the preparation.
Cerebrolysin comes in injections at 2ml, 5ml, 10ml, and 20ml ampoules. Unfortunately, there is no such thing as Cerebrolysin tablets or nasal spray. Cerebrolysin injections have been thoroughly studied over the years. Extensive research includes their oligopeptide and membrane fractions, vitamin activity, amino acids, trace element composition, and the effect on the homeostasis of brain trace elements and, most importantly, its peptide composition.
CEREBROLYSIN DRUG STUDY AND HISTORY
Cerebrolysin was developed back in 1949 by an Austrian professor Gerhart Harrer. He discovered that during the enzymatic hydrolysis of brain tissue, substances that have a stimulating effect on nerve cells and that regulate autonomic disorders are formed.
The first publications on the clinical use of Cerebrolysin date back to 1954-1955, when the arousing effect of the drug was discovered in patients with hypoglycemic coma [1]. In a series of studies on 45 patients, half of them arose from coma immediately after the drug administration. Evaluation of electroencephalogram (EEG) parameters showed the disappearance of pathological changes characteristic of hypoglycemia.
In subsequent years, the number of publications on the clinical use of Cerebrolysin injections increased: the treatment of mental disorders of various natures, cerebrovascular disorders, chronic brain injuries, cerebral atherosclerosis etc.
In the 1950s the nerve growth factor (NGF) was discovered. It explained the therapeutic mechanism of Cerebrolysin. Later studies showed that a pharmacological effect resembling that of the NGF could persist for 24 hours after the administration of Cerebrolysin.
After many success stories, extensive research, and the discovery of neurotrophic regulation, the popularity of the drug has increased drastically. Now it can officially be used as a treatment of various cerebral disorders in more than 50 countries, mostly in Europe and Asia.
WHAT IS CEREBROLYSIN USED FOR?
Cerebrolysin injections are used in the following cases:
- Strokes and stroke complications,
- Alzheimer’s disease and other types of dementia,
- Traumatic brain injuries (TBI),
- Spinal cord injuries,
- ADHD in children,
- Antidepressant-resistant depressions,
- Other cerebral diseases.
In Austria, the homeland of Cerebrolysin, it is included in the Neurorehabilitation after stroke guidelines. In the US, Cerebrolysin has the status of a new drug approved by the FDA for private clinical use. Cerebrolysin is also approved in Canada. In Russia (USSR) it has been used since the early 1970s and has been included in the List of Vital & Essential Drugs since 1992.
CEREBROLYSIN IN THE TREATMENT OF DEMENTIA
In the treatment of dementia, early diagnostics of even a mild cognitive impairment (MCI) is of utmost importance. It is believed that an MCI is the earliest stage of dementia. People with MCI may have some difficulties with memory and trouble recalling some words, but they manage their everyday routine well. However, 70% of those diagnosed with MCI progress to dementia or Alzheimer’s disease (AD) at some point.
Cerebrolysin peptide can slow down the progression of the disease. In Russia, for example, it was included in the Anti-dementia plan for 2025.
The Cochrane Library published a critical study of Cerebrolysin in vascular dementia in 2013 and then again in 2019 [27]. Six randomized controlled trials with a total of 597 participants from China, Russia, and Romania with mild to moderate dementia were included in the study. It was stated that it might have positive effects on the improvement of cognitive function and global function in older patients with vascular dementia of mild to moderate severity. But scientists were quite skeptical and stressed that further trials need to be conducted in order to provide recommendations for treatment.
Another complex study investigated Cerebrolysin in mild to moderate Alzheimer’s disease (AD). All randomized double-blind placebo-controlled studies on 30 ml/day of Cerebrolysin in mild-to-moderate AD were included. This meta-analysis provided evidence that Cerebrolysin has an overall beneficial effect and a favorable benefit-risk ratio in patients with mild-to-moderate AD. [22]
CEREBROLYSIN IN TBI TREATMENT
The research results have shown the high efficiency of the administration of Cerebrolysin peptide in patients with acute TBI. The use of Cerebrolysin injections in patients with acute TBI was based on an increase in the activity of superoxide dismutase and, as a consequence, a sharp decrease in the activation of peroxidation.
142 patients were included in the CAPTAIN II study (out of 187 screened patients) [30]. The CAPTAIN II study shows that after moderate to severe TBI, Cerebrolysin treatment improves the overall outcome at day 90 compared to placebo, confirming the results of a previous study conducted in a sample of Asian patients [21]. It is extremely important that the clinical efficacy and safety of the use of high doses of Cerebrolysin injections have been proven in comparison with the previously accepted doses of 1, 2, 5, and 10 ml in clinical practice. The introduction of Cerebrolysin in high doses (50 ml) made it possible to achieve better results in the treatment and rehabilitation of patients with moderate and severe TBI. [29] As practice shows, repeated and long-term courses of treatment with Cerebrolysin peptide lead to an increase in the effectiveness of therapy, especially in relation to cognitive and behavioral functions.
CEREBROLYSIN IN STROKE TREATMENT
Cerebrolysin peptide is recommended in the stroke treatment guidelines of top reference countries (Austria, Canada, and Germany). For example, in the Austrian guidelines, it is suggested that Cerebrolysin may have a positive effect on the restoration of the upper extremities after stroke if taken at the dose of 30 ml for 3 weeks and longer. Cerebrolysin in Canada is said to be beneficial for aspects of upper limb function following stroke and is described and recommended in the Evidence-Based Review of Stroke Rehabilitation (EBRSR) by Robert Teasell, MD et al [25] under the Canadian Partnership for Stroke Recovery (CPSR). Its efficacy has been proven in 36 double-blind studies and trials with more than 5.000 patients.
In 2017 a group of scientists combined the results of two prospective, randomized, double-blind, placebo-controlled studies (CARS-1 and CARS-2) concerning the efficacy of Cerebrolysin on motor recovery during early rehabilitation after stroke. [24] Treatment with 30ml of Cerebrolysin once a day for 3 weeks was started 24-72 h after the stroke onset. In addition, patients participated in a standardized rehabilitation program. The results of the study showed that Cerebrolysin had a beneficial effect on motor function and neurological status in early rehabilitation patients after acute ischemic stroke. Safety aspects were comparable to placebo, showing a favorable benefit/risk ratio.
Cerebrolysin peptide stimulates natural recovery processes after stroke. These processes are most prominent during the first 3 months post-stroke. Both research and clinical data indicate that the best treatment effects are seen when Cerebrolysin is administered as soon as possible after the onset of the disease and the treatment continues during the natural recovery phase.
Combining Cerebrolysin treatment with a standardized rehabilitation program may have a potential synergistic effect in the subacute stage of stroke (check more).
CAN I USE CEREBROLYSIN TO TREAT ANOSMIA?
There is a recent study by Sherifa Ahmed Hamed [31] where the researchers hypothesize that Cerebrolysin, a drug of neurotrophic and neuroprotective properties, can be used to treat patients with persistent post-COVID anosmia or ageusia or promote functional recovery of smell and taste deficits.
Cerebrolysin was prescribed in a dose of 10 ml ampoule once daily through intramuscular injection five times per week, for a total of 20 treatments (for 4 weeks), after which the cycle was repeated again for at least 8 more weeks. The study ended in March 2021 and the results are still to be published which does not allow us to assume yet that it indeed may have a beneficial effect in regards to Covid-2019.
WHAT IS CEREBROLYSIN COMPOSITION?
The exact composition of Cerebrolysin is still unknown. Studies have shown that purified Cerebrolysin contains more than 100 oligopeptides and protein motifs with a molecular weight of up to 5800 Da.
PEPTIDES IN CEREBROLYSIN
The result of the Cerebrolysin composition research was the discovery of oligopeptides vital for the neurochemistry of the brain.
The drug contains the tripeptide Glu-Cis-Gly (glutathione). It is a widespread antioxidant oligopeptide that is present in all tissue formations, adrenal glands, erythrocytes, cerebrospinal fluid, brain, and the lens of the eye. Its participation in redox reactions is of great importance [13].
Cerebrolysin nootropic contains the tripeptide thyroliberin with the amino acid sequence Glu-His-Pro, which is an antagonist of opioid activity. The main function of this oligopeptide is to enhance the secretion of thyrotropin by the anterior pituitary gland, as well as to stimulate the growth hormone corticotropin. [20]
An enkephalin motif with the amino acid sequence Tyr-Gly-Gly-Phе was also isolated from the lipid-free Cerebrolysin samples. Enkephalins are peptides with a wide range of action in the CNS and the peripheral nervous system. They can regulate pain sensitivity, sexual behavior, motivation for satisfaction, and adaptation processes [13].
A stable collagen motif Gly-Pro-Hyp of the brain supporting proteins was also found in Сerebrolysin. This motif can be used to reconstruct damaged and synthesize new supporting collagen and other proteins. [20]
The presence of active peptide fragments of NGF peptides, enkephalins, orexin, and galanin in the preparation was established as well. [18] With the exception of the ser-ser-phe-gly-ile fragment (corresponding to ABC-transport proteins), all identified peptides in the studied samples of Сerebrolysin are fragments of human proteome neuropeptides.
Cerebrolysin contains the PQRF peptide, which corresponds to a fragment of the recently discovered neuroactive neuropeptide VF. Unfortunately, there is still little data on the biological role of this neuropeptide. Although it is known that it can activate mu-opioid and kappa-opioid receptors and regulate the hypothalamus [15].
The cys-cys-arg-gln-lys (CCRQK) peptide contained in the studied samples of Сerebrolysin is identified as a fragment of the orexin polypeptide (ORX gene). The CCRQK peptide, according to integral bioinformatics analysis, can be important for the interaction of orexin-A with receptors. Orexins has an impact on a variety of human physiological functions, including energy metabolism, hormone balance, and fluid regulation in the body. Orexin-A also increases the expression level of NT-3 [16], which in turn supports the survival and differentiation of neurons, also stimulating the formation of new neurons and synaptogenesis.
Galanin in Cerebrolysin compositionacts primarily as a neuropeptide that modulates the secretion of the most important neurotrophic neurotransmitters acetylcholine, serotonin, and norepinephrine. The neurotrophic effects of galanin are being actively studied at the present time. Galanin is essential for the development and functioning of neurons; it stimulates the “sprouting” of axons [14].
VITAMINS IN CEREBROLYSIN
Since Cerebrolysin is obtained by enzymatic fractionation of the brain extract of young pigs, which is characterized by a high antioxidant potential, it has been suggested that Cerebrolysin also has vitamin-like activity (in particular, vitamins B1, B12, E, and folates), as well as superoxide dismutase activity.
Particular attention is drawn to the activity of vitamin B12 (cyanocobalamin). The presence of a stable high activity of cyanocobalamin in Сerebrolysin proves the preservation of the bio coordination bond of the cyano group with cobalt, which is fundamentally important for the full development of the neurotrophic effects of vitamin B12. Cyanocobalamin not only inhibits fatal damage to the cerebral cortex but also, by participating in the synthesis of choline and methionine, has a beneficial effect on the liver, preventing the development of fatty hepatosis (in particular, in alcoholism). The indicated properties of cyanocobalamin can also explain the positive effect of long-term use of high doses of Cerebrolysin. [31].
Vitamin B1 (thiamine) is characterized by the most stable activity and high concentration in the preparation. In addition to the well-known enzymatic function, vitamin B1 controls the transport of Na+ ions across the neuron membrane, it participates in the exchange of acetylcholine, and conduction of nerve impulses. Therefore, it has a status of the neurotransmitter [5].
AMINO ACIDS IN CEREBROLYSIN
The drug is strictly standardized in terms of the content of amino acids (alanine, arginine, aspartic acid, valine, histidine, glycine, glutamic acid, isoleucine, leucine, lysine, methionine, proline, serine, threonine, tryptophan, phenylalanine) in 1 ml of a ready-to-use solution.
Amino acids play the role of a “building material” for the synthesis of all proteins. In addition, they are characterized by a number of physiological functions due to their chemical structure and biochemical properties. For example, on the basis of 20 “proteinogenic” (i.e., used to build proteins) amino acids, “non-protein” amino acids are synthesized. These “non-protein” amino acids (carnitine, taurine, gamma-aminobutyric acid – GABA – and dopamine) are also important for the functioning of the nervous system.
From a neurochemical point of view, amino acids can be subdivided as follows:
1) exciting (glutamate, aspartate);
2) inhibitory (GABA, beta-alanine, taurine, glycine);
3) neutral (lysine, arginine).
As a result, amino acids can regulate all the main nervous processes [28]: arousal and inhibition, wakefulness and sleep, aggression and anxiety, synaptic plasticity, emotions, behavior, memory, and learning. Considering that the bioavailability of amino acids, when administered intravenously, is close to 100%, it becomes clear that Cerebrolysin is an essential source of easily available amino acids necessary for brain recovery.
MICRO ELEMENTS IN CEREBROLYSIN
The preparation contains trace elements that play an important role in the functioning of a number of enzymes (sulfur, phosphorus, potassium, sodium, calcium, magnesium), and essential trace elements (lithium, selenium, zinc, tin, cobalt, silicon, iron, copper, manganese, chrome, vanadium).
Cerebrolysin is distinguished by superoxide dismutase activity. And this activity is ten times higher than that of a number of other neuroprotective drugs used in clinical practice (bilobil, actovegin, cerebrolysat) [20].
MECHANISM OF ACTION OF CEREBROLYSIN
Cerebrolysin is a neurotrophic peptide drug with multimodal pharmacological properties. It is indicated for the treatment of acute and chronic CNS disorders. The pathophysiological mechanisms targeted by Cerebrolysin belong to two distinct categories:
- The support of endogenous repair and recovery processes as a consequence of injury or degenerative disease;
- The protection against pathological events and cascades caused by an injury or a degenerative disease.
The natural repair and recovery processes in the CNS start immediately upon injury and play an important role in the continuous defense against neurodegeneration in chronic CNS disorders (e.g. Alzheimer’s disease).
Cerebrolysin injections were shown to modify two major signaling pathways: the neurotrophic factor (NTF) and the sonic hedgehog (Shh) signaling pathway. These pathways regulate the cellular processes of neurogenesis, angiogenesis, dendritic arborization, axonal sprouting, myelination, and remodeling of the neurovascular unit on a molecular level, thereby supporting the maintenance and repair of the neuronal network.
The pathological events and cascades after stroke or trauma lead to secondary injuries, which further compromise the motor and cognitive functions of a patient. Among the most relevant molecular processes targeted by Cerebrolysin peptide in the acute phase of an injury are events of the ischemic cascade, like:
- Excitotoxicity,
- Uncontrolled apoptosis,
- Overactivation of proteolytic enzymes,
- Overproduction of reactive oxygen species (ROS).
In the early post-acute phase, Cerebrolysin prevents the formation of toxic protein aggregates (amyloidosis) and lowers the level of inflammatory processes.
The low molecular weight composition of Cerebrolysin peptide allows it to relatively easily pass through the blood-brain barrier (BBB) and go directly to the nerve cells. This is the difference between the action of Cerebrolysin and experimentally used drugs such as NGF (nerve growth factor), BDNF (brain-derived nerve factor), etc., large molecules of which cannot cross the BBB. As a result, they have an extremely low therapeutic potential compared with Cerebrolysin. Here is a Cerebrolysin review on YouTube by Lucas Aoun with a detailed explanation of how it works in the brain ⬇️
SAFETY PROFILE OF CEREBROLYSIN
Cerebrolysin injections are safe and well tolerated. The experience with Cerebrolysin during many years of clinical application, the information from post-marketing surveillance studies, and the safety data from double-blind, placebo-controlled clinical trials indicate a high degree of safety (CASTA study). According to the EMA classification, Cerebrolysin peptide is in the “SAFE” category.
Please note that maintaining the sterile process of administration is crucial for the safe use of Cerebrolysin. There is more information on how to use Cerebrolysin down below.
CAN CEREBROLYSIN BE USED IN PEDIATRICS?
Interestingly, Cerebrolysin unique features encourage pediatricians to consider its use in children. In recent years it is being more and more prescribed for the treatment of neurological conditions in younger patients.
The clinical efficacy of Cerebrolysin peptide in children was confirmed by Medvedev MI et al [19] in a study of premature infants with perinatal hypoxic-ischemic brain damage. After the treatment, the indicators of the muscular-forward tonus and reflexes, as well as the formation of bioelectric activity of the brain significantly improved.
WHAT ARE CEREBROLYSIN SIDE EFFECTS?
In general, reported adverse drug reactions from Cerebrolysin-treated patients are transient and mild in intensity. Most frequently reported adverse reactions are:
- dizziness,
- headache,
- sweating,
- nausea.
CEREBROLYSIN DRUG INTERACTIONS
Given the pharmacological profile of Cerebrolysin injections, special attention should be given to possible additive effects when co-prescribing it with antidepressants, including MAOIs (monoamine oxidase inhibitors). In such cases, it is recommended to reduce the dosage of the antidepressant.
Do not mix Cerebrolysin peptide and balanced amino acid solutions in a single solution for infusions. Cerebrolysin is also incompatible with solutions containing lipids or modifying the pH of the medium (5-8).
The compatibility of the preparation with the following standard infusion solutions was checked and confirmed (for 24 hours at room temperature and with illumination):
- 0.9% solution of sodium chloride (9 mg NaCl / ml);
- Ringer’s solution (Na+ – 153.98 mmol/l, Ca2+ – 2.74 mmol/l, K+ – 4.02 mmol/l, Cl- –163.48 mmol/l);
- 5% glucose solution.
It is permissible to use Cerebrolysin simultaneously with vitamins and preparations for cardiac circulation improvement. But these preparations should NOT be mixed in the same syringe with Cerebrolysin.
CAN CEREBROLYSIN CAUSE PRION DISEASE?
Some people are concerned about whether it is possible to get infected with prions by taking medications of animal origin (Cortexin, Solcoseryl, Cerebrolysin, etc). First of all, we would like to provide a short description of what the prion disease is.
PRION DISEASE – WHAT IS IT?
Prion diseases (PD), or transmissible spongiform encephalopathies (TSE), are a group of neurodegenerative disorders characterized by rapidly progressive dementia and movement disorders. The term “prion” appeared in the middle of the 20th century. The nature of spongiform encephalopathy remained unclear for a long time.
In the 60s, British researchers T. Alper and J. Griffith hypothesized that some TSEs are caused by pathogens composed exclusively of proteins. In 1982 S. Prusiner isolated this agent from the brains of sick animals and then studied its properties. The infectious agent consisted of one protein. Based on the experimental data, the concept of prion proteins was formulated.
WHAT CAUSES PRION INFECTION?
All mammalian prion diseases known to date are caused by the PrP protein. Its form with a normal tertiary structure is designated as PrPC (“C” for common or cellular). The pathological form of the infectious protein is called PrPSc (“Sc” for scrapie-sheep pruritus, after the name of one of the first diseases with an established prion nature) or PrPTSE (“TSE” for Transmissible Spongiform Encephalopathies).
There are three variants of the occurrence of prion diseases:
- direct infection,
- hereditary and
- sporadic (arising spontaneously) forms.
Infection of both humans and animals (mainly horned cattle) is most often alimentary (through the gastrointestinal tract), i.e. the main way of acquiring the disease is through the consumption of contaminated foods.
Prion infection can also occur in case of the use of non-sterile surgical instruments. If contaminated material gets on the skin, it is first disinfected with a 4% sodium hydroxide solution for 5-10 min, and then washed with running water [26]. In fact, Cerebrolysin has sodium hydroxide in its composition as an excipient.
There are no treatments for PD today. Prescriptions are limited to supportive care. Genetic counseling is recommended for relatives of patients with a family history of PD. The development of special vaccines for animals is underway, which in the future should help in the creation of a vaccine against PD for humans.
IS THERE A RISK OF PRION INFECTION WITH CEREBROLYSIN?
There are a lot of medications that are produced from animal tissues in modern medicine. Is there a potential risk of PD for patients using them?
First of all, it is important to note that Cerebrolysin is derived from pig brain only (industrially since 1943), in which no prion diseases have been identified so far. Besides, the biological safety of the products based on animal tissues from prion contamination is ensured by specially developed procedures that meet the requirements of the European Medicines Agency (Note for Guidance 410/01), as well as the requirements of the European Pharmacopoeia (Eur. Ph. 7.0, 01/2008: 50107, clause 5.1.7 “Virus safety” and Eur. Ph. 7.0, 01/2008: 50208, clause 5.2.8. “Minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products”).
Raw material taken from young healthy animals undergoes the most stringent selection and is used only after confirming viral safety and obtaining a veterinary certificate. The production of Cerebrolysin is based on modern technological processes aimed at isolating low-molecular water-soluble peptides from the tissues of raw materials. The process makes it possible to completely exclude peptides with a molecular weight of more than 10 kDa from the final preparation, while, as known, the molecular weight of prions is 33–35 kDa. [26] It is important to note that the substance of the drug (Cerebrolysin dry extract) is only an intermediate product and it is not directly used for the preparation of the sterile drug. It is processed at additional technological stages. All these facts eliminate the possibility of prion infection with Cerebrolysin.
WHAT ARE CEREBROLYSIN DOSAGE RECOMMENDATIONS?
The most common Cerebrolysin protocol for improving brain function is IM injections of 5 ml daily for 4 weeks, unless otherwise prescribed by your attending physician. The medicine is best taken in the first half of the day.
It is recommended to start Cerebrolysin therapy as early as possible. Cerebrolysin is supposed to be used as an adjunct to the standard treatment. Dose recommendations from the manufacturer are the following:
| Condition | Daily dosage | Initiation of treatment | Duration of treatment |
| Stroke | 20 – 50 ml | asap | 10 – 21 days |
| Vascular dementia | 10 – 30 ml | asap | 1 cycle: 5 days a week/ 4 weeks 2-4 cycles per year In the next courses of therapy, the frequency of use can be reduced from daily applications to 2-3 applications per week. |
| Alzheimer’s disease | 10 – 30 ml | asap | 1 cycle: 5 days a week/ 4 weeks 2-4 cycles per year In the next courses of therapy, the frequency of use can be reduced from daily applications to 2-3 applications per week. |
| Traumatic brain injury (TBI) | 20 – 50 ml | asap | 7 – 30 days |
Route of Administration
IV infusion | 10ml – 50ml | diluted to at least 100ml total volume with Saline, Ringer solution, or 5% glucose solution | Infuse slowly within 15 minutes |
IV injection | up to 10ml | undiluted | inject slowly for 3 minutes |
IM injection | up to 5 ml | undiluted | inject slowly for 3 minutes |
HOW TO INJECT CEREBROLYSIN?
The main ways of administering the medication, which are indicated in the official instructions are intramuscular (IM) and intravenous (IV). All manipulations are carried out in gloves. First, you need to choose the site where the injection will be made:
- Hip,
- Deltoid,
- Femoral, front thigh.
It is preferable to choose the gluteal region if you do not have enough experience. The buttock is mentally divided into four squares. The injection is placed in the upper outer square. It is better to alternate injection sites. For example, if yesterday you made an injection in the right buttock, today – do it in the left one.
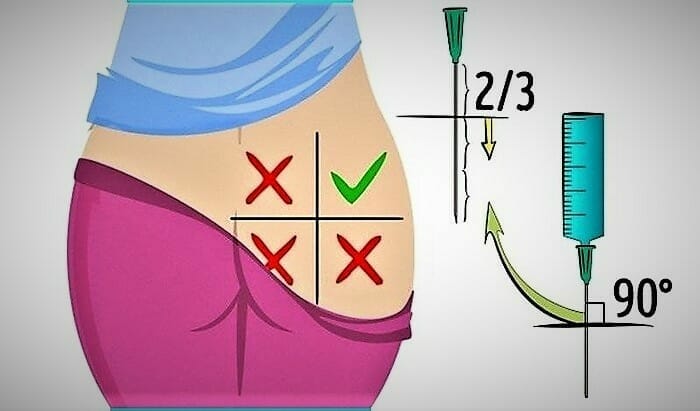
- Before injection, treat the selected site with a cotton swab dipped in medical alcohol. Wipe in a circular motion from center to the periphery.
- Before starting the procedure, tap the syringe slightly, lifting it with the needle up. Then press the plunger until all the air is out of the syringe.
- Stretch the skin at the site of the intended injection with your hand. Take the syringe in another hand, lift it up a little, and sharply insert the needle at 2/3 of its length. During injection, insert the needle perpendicular to the surface of the body.
- The needle introduction shall be quick. If you insert the needle slowly, it will be more painful.
- Then press down on the plunger to slowly get the medicine into the muscle. The optimal injection rate is 1 ml in 10 seconds.
- After that, quickly remove the needle and put the cap on it. Only in this form can the syringe be thrown away.
- It is recommended to massage the injection site so that the drug is better absorbed into the tissue. Re-treat the area into which the injection was made with a cotton swab dipped in an alcohol solution. This is done in order to prevent infection.
- The IV and IM injection have to be administered immediately after opening the ampoule! Do not store the solution. Start the infusion as quickly as possible after the dilution.
WHAT IS THE BEST SIZE OF THE NEEDLE FOR CEREBROLYSIN INJECTION?
There are no specific recommendations from the producer of Cerebrolysin or Cortexin on what needle size to choose because it highly depends on the spot you want to inject it in and the person’s complexion. However, the most commonly recommended needle size for intramuscular injections ranges from 18G to 25G. The injections are to be made slowly.
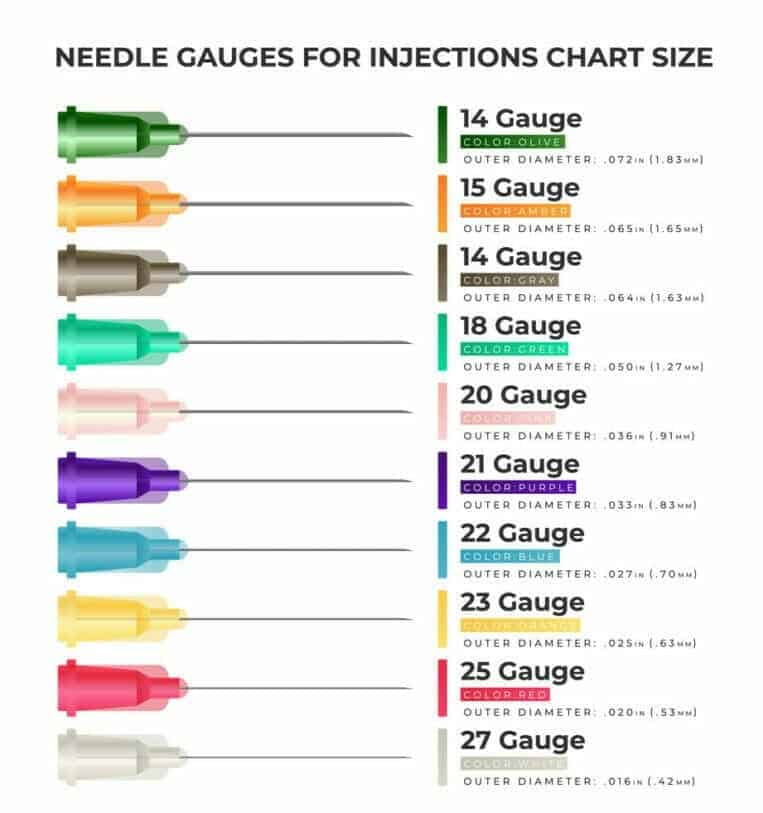
Needle gauges for injections chart size. Source: VectorStock.com/25936567
SHOULD I USE FILTERED NEEDLES WHEN INJECTING CEREBROLYSIN?
Some people are concerned that when breaking an ampoule small pieces of glass may get into the solution. EverPharma, the producer of Cerebrolysin does not give any specific recommendations regarding the use of filtered needles when aspirating Cerebrolysin from its glass ampoules. However, if you are worried about glass particle contamination, then filter needles are the most reliable precaution you can take.
But bear in mind that this type of needle is more expensive compared to regular ones. As a less costly option, you may simply want to consider using regular needles of smaller diameters.
STERILITY PRECAUTIONS
Special precautions to guarantee the sterility must be taken during the dilution and administration of Cerebrolysin:
- Remove the solution from an ampoule immediately prior to use;
- Do not leave an open ampoule on the treatment table;
- Always use disposable one-way IV infusion sets and cannulas;
- When Cerebrolysin is administered via a long-term IV catheter, the catheter has to be rinsed before and after the application with physiological sodium chloride solution;
- Pay special attention to recommended infusion/injection times.
CAN I STORE AN OPEN VIAL OF CEREBROLYSIN? CAN I SPLIT THE CONTENTS OF THE VIAL?
There are many anecdotal reports including quite trustworthy ones, TheLongLived for example, of people splitting the contents of the vial and storing them for multiple uses.
Some customers consider it economically feasible to buy Cerebrolysin 10ml ampoules and then split them into capped syringes and store them in the fridge for 24h, without freezing.
Prior to an injection the syringe is taken out of the fridge and warmed at room temperature. For sterility purposes the needle can be changed too. You can read more about it on Reddit. However, please be aware of the fact that according to the official instructions it is recommended to use the entire solution of the vial as soon as you open it. And it is not recommended to store an open ampoule. In that case Cerebrolysin can lose some of its potency, because it gets rapidly oxidized.
HOW TO MAKE AN IV INFUSION?
Cerebrolysin intravenous infusion is the fastest way of getting the medication into the bloodstream. That is why some people lean towards this mode of administration rather than IM. There are many videos on YouTube on how to do it. Specialized medical kits would be needed to conduct the procedure at home.
However, if you want to perform Cerebrolysin infusion intravenously, the best option would be turning to professional medical aid.
HOW TO MAKE A CEREBROLYSIN NASAL SPRAY?
There are some anecdotal reports of people who make Cerebrolysin nasal spray forms for better convenience: Intranasal Cerebrolysin/Cortexin: A Reddit Tutorial.
There were also some negative anecdotal reports of those who tried Cerebrolysin intranasal route of administration in pursuit of avoiding unpleasant injections. It should be noted that they reported serious and long-term and/or permanent adverse reactions of Cerebrolysin nasal spray.
That is why we strive to promote the on-label use of the pharmaceutical products and following the Cerebrolysin official instructions if you want to minimize risks and maximize benefits at the same time.
HOW TO MAKE A CEREBROLYSIN ORAL SOLUTION?
Just like many other drugs which are popular among nootropic enthusiasts around the world, the conventional form of Cerebrolysin peptide is being modified and adapted to better fit the purposes of the customers.
Here is an anecdotal report and discussion devoted to an oral version of Cerebrolysin. It is supported by a paper [7] that has studied the effects of a single oral dose of Cerebrolysin solution (30 ml) on brain bioelectrical activity and on cognitive performance in healthy elderly people. The results of the study suggested that oral Cerebrolysin might be useful for the treatment of memory impairment and brain damage in elderly subjects with or without neurodegenerative disorders.
However, instead of testing unconventional ways of Cerebrolysin administration, it might be a better option to consider safe alternatives ⏬
WHAT ARE ALTERNATIVES TO CEREBROLYSIN?
CEREBROLYSIN VS CORTEXIN
Cortexin ® is a mixture of neuropeptide fractions, amino acids, vitamins, and minerals, all of which have a pronounced but subtle positive effect on the brain. Similar to Cerebrolysin, Cortexin is based on neuropeptides and amino acids. However, there is a difference: Cortexin has a significantly larger amount of peptide fractions and fewer amino acids. While the former is produced from pig brains only, the latter is extracted mostly from young horned cattle cerebral cortex by acetic acid extraction. Due to its high safety profile, Cortexin is suitable for all ages and is often prescribed for children at lower doses (5mg). However, in case of Cortexin mild allergic reactions are more possible compared to Cerebrolysin peptide.
CAN CORTEXIN AND CEREBROLYSIN BE USED IN A STACK?
Experts do not consider it effective to combine these drugs. But quite often they recommend using them in succession or alternating the courses. This is because Cerebrolysin and Cortexin have different ratio and composition of the peptides in them.
Dr Alexander Galushchak is talking about how autism may be treated with neuro stimulants and nootropics, including Cerebrolysin and Cortexin ⬇️
CEREBROLYSIN VS CELLEX
Cellex ® is a neuroprotector for the treatment of acute disorders of the cerebral circulation. It consists of a balanced and stable mixture of biologically active proteins and polypeptides.
Both Cellex and Cerebrolysin preparations come in injection form. But unlike Cerebrolysin which is extracted from the brain of young pigs, Cellex is produced from pig embryonic brain tissue, which is why the concentration of neuropeptides in it is higher. For these reasons Cellex is more expensive than Cerebrolysin.
MEMOPROVE® (N-PEP 12) – A CEREBROLYSIN PILL
N-PEP-12 is a pig brain-derived peptide blend obtained by enzymatic hydrolysis of purified proteins of nerve cells. The mechanism of action of Memoprove is directed at stimulation of neuroplasticity and neuroprotection.
The composition of Memoprove is very similar to that of Cerebrolysin. Some even call it Cerebrolysin pills. Its efficacy is confirmed by preclinical studies, as well as by the results of placebo-controlled studies in groups of volunteers [11, 12]. Memoprove also has a favorable safety profile.
Please note that Memoprove is a food supplement that is not designed to treat any disease. And it is known to be less effective compared to Cerebrolysin peptide.
OTHER CEREBROLYSIN ANALOGUES
Cerluten® is a cytomaxe which means that it is a natural peptide, derived from the animal brain cortex and designed to protect, restore and improve functions of the central nervous system and the brain.
There are also Ukrainian and Belorussian generics similar to Cerebrolysin produced under the brand name Ceregin® and Cerebrolysat®. And they are also derived from the brains of cattle and porcine.
However, Cerebrolysin is considered to be of higher quality and safety compared to its generics. And if we are talking about the products extracted from raw tissues, the technologies, and purification process play a crucial role in the production of medication.
CEREBROLYSIN VS P21
What is the difference between Cerebrolysin and the P21 peptide? Should one choose a nasal spray over an injection? – Well, actually it is not quite right to compare these two products. Let us see why.
On the one hand there is P21 which is a peptide that is artificially synthesized and is not used as a medicine in any country. One might also want to take caution with intranasal RoA as there is some negative feedback on that.
On the other hand, there is Cerebrolysin which is a complex of essential substances including P21. Besides, it also contains neuropeptides, amino acids, and minerals. It is a very reliable and safe drug that has been successfully used in many countries for several decades.
EVER PHARMA, CEREBROLYSIN MANUFACTURER
About the company
The company was founded under the brand name EBEWE Pharma in 1934 in Vienna, Austria. Later, the company became independent EVER Pharma GmbH. Today the main factory is located in Jena, Germany. It has many years of experience in such a complex industry as the development and production of sterile dosage forms and is considered a recognized partner in the pharmaceutical manufacturing industry.

Products
EVER Pharma offers high-quality products in the field of neurology and emergency conditions. The product range includes drugs for the treatment of stroke, head injuries, vascular dementia, Alzheimer’s disease, Parkinson’s disease, hypertension, and epilepsy. The company also provides clinically approved nutritional supplements to support memory function and enhance mental performance.
Here is an original 84-page Monograph about Cerebrolysin by EVER Pharma.
License
The drugs fully comply with the requirements of international pharmaceutical quality standards. The company has a license of Good Manufacturing Practice (ЕU GMP ISO 13485:2013, ISO 9001:2008).
IS MY CEREBROLYSIN AUTHENTIC?
Make sure to buy Cerebrolysin from a trustworthy vendor. You can verify the authenticity of your Cerebrolysin by sending an email via Cerebrolysin EverPharma official website. Alternatively you can contact us at [email protected] and our customer support will assist you.
Can CosmicNootropic provide the CoA of Cerebrolysin?
Yes. Please find the Certificate of Analysis (CoA) of Cerebrolysin 2ml, 5ml, and 10ml below.
IS IT NORMAL THAT THE SEAL ON THE CEREBROLYSIN BOX IS OPEN?
The box has a dotted-cut narrow film. It does not have protective or verifying purposes. And it is very fragile. In fact until the parcel gets to the final point of destination it goes through many stages: from production and distribution to shipment to the end-buyer address. During this long journey, the delicate film can easily be tampered or torn at the slightest pressure. This does not affect the quality of the product or the integrity of the ampoules in the box.
Besides, the individual packaging of the ampoules, the secondary packaging, is also sealed profoundly. Please check the photo below.

Cerebrolsyin individual packaging
WHERE CAN I BUY CEREBROLYSIN?
There are several companies where you can buy original Cerebrolysin, for example CosmicNootropic. It is a reliable nootropics vendor which has been around since 2015. We offer the most popular forms: Cerebrolysin 5ml, Cerebrolysin 10ml ampoules as well as other volumes.
ANECDOTAL CEREBROLYSIN REVIEWS
There are many anecdotal reports on the use of Cerebrolysin on Reddit. This is probably one of the most comprehensive Cerebrolysin reviews where a person explains the positive sensations and the mode of administration, and compares Cerebrolysin to other nootropics like Phenylpiracetam, Phenibut or Noopept.
In another detailed Cerebrolysin review a person describes the positive manifestations and a few drawbacks on a daily basis. There are of course various reports on different stacks with Cerebrolysin. Here a person stacked Cerebrolysin injections and Buthylphthalide with some positive feedback and dosage recommendations.
Of course, there are also lots of experiences on Cerebrolysin product page at CosmicNootropic. Please feel free to indulge in a discussion devoted to Cerebrolysin and share your experience.We like reading and answering your comments very much!
CONCLUSION
All in all, Cerebrolysin is a very potent peptide with lots of positive review. But significant progress in understanding the physiological effects of Cerebrolysin, there are a number of pressing issues related to clarifying its composition. Hence, further in-depth study of Cerebrolysin is important. That would allow for a much deeper understanding of the mechanism of action of the drug and expand the scope and methods of its use.
Cerebrolysin can be of help in the treatment of serious neurologic conditions such as stroke, dementia and TBI. Considering all the indications for use, it is still important to be aware of the fact that any recovery requires a multimodal approach including physical activity, physiotherapy, and medical therapy combined together. Cerebrolysin can be of major help but one should not regard it as a magic shot that would do wonders.
Feel free to indulge in a discussion devoted to Cerebrolysin on Reddit. We like reading and answering your comments very much!
Bibliography
- H Hetzel, E Niedermeyer (1955). Arousing effects of brain hydrolysate in hypoglycemic coma and their electroencephalographic appearance. https://pubmed.ncbi.nlm.nih.gov/13249577/
- Dubenko AE (1991). The role of lipid peroxidation and the activity of energy metabolism enzymes in the pathogenesis of acute closed craniocerebral trauma. https://pubmed.ncbi.nlm.nih.gov/1792780/
- Akai F., Hiruma S. (1992). Neurotrophic factor-like effect of FPF 1070 on septal cholinergic neurons after transections of fimbria-fornix in the rat brain. https://pubmed.ncbi.nlm.nih.gov/1515704/
- De Wied D. (1997). Neuropeptides in learning and memory processes. https://pubmed.ncbi.nlm.nih.gov/9062665/
- Gromova OA et al (1998). Effects of cerebrolysin on the oxidant homeostasis, the content of microelements and electrolytes in children with minimal brain dysfunction. https://pubmed.ncbi.nlm.nih.gov/9505400/
- Leppala JM et al (1999). Different risk factors for different stroke subtypes. https://pubmed.ncbi.nlm.nih.gov/10582974/
- Alvarez XA et al (2000). Oral Cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. https://pubmed.ncbi.nlm.nih.gov/10961443/
- Goldstein LB et al (2001). Primary prevention of ischemic stroke: a statement for healthcare professionals from the Stroke Council of the American Heart Association. https://pubmed.ncbi.nlm.nih.gov/11136703/
- Plum F (2001). Neuroprotection in acute ischemic stroke. https://pubmed.ncbi.nlm.nih.gov/11277833/
- Gomazkov OA (2005). Neurotrophic and growth factors of the brain: regulatory specificity and therapeutic potential. https://pubmed.ncbi.nlm.nih.gov/15909662/
- Volc D, Alvarez XA, Moessler H (2005). Cognitive Effects of the Novel Neuroprotective Dietary Supplement N-PEP-12: Evidence from a Self-Assessment Study. https://www.xediton.com/wp-content/uploads/2017/05/Volc-et-al.pdf
- Windisch M et al (2005). N-PEP-12™ – a novel peptide compound that protects cortical neurons in culture against different age and disease associated lesions. https://pubmed.ncbi.nlm.nih.gov/15750682/
- Gromova OA et al (2006). An oligopeptide membrane fraction of cerebrolysin. https://pubmed.ncbi.nlm.nih.gov/16921723/
- Suarez V et al (2006). The axotomy-induced neuropeptides galanin and pituitary adenylate cyclase-activating peptide promote axonal sprouting of primary afferent and cranial motor neurones. https://pubmed.ncbi.nlm.nih.gov/17004919/
- Cline MA et al (2008). Short-term anorexigenic effects of central neuropeptide VF are associated with hypothalamic changes in chicks. https://pubmed.ncbi.nlm.nih.gov/18540998/
- Yamada N et al (2009). Orexins increase mRNA expressions of neurotrophin-3 in rat primary cortical neuron cultures. https://pubmed.ncbi.nlm.nih.gov/19026718/
- Bin Li et al (2010). Neurotrophic peptides incorporating adamantane improve learning and memory, promote neurogenesis and synaptic plasticity in mice. https://www.sciencedirect.com/science/article/pii/S001457931000520X
- Torshin IYu, Gromova OA (2012). Expert data analysis in molecular pharmacology. https://artlib.osu.ru/web/books/content_all/4911.pdf
- Medvedev MI et al (2014). Clinical and neurophysiological objectification and evaluation of treatment efficacy in children with perinatal hypoxic-ischemic injury of the CNS. https://pubmed.ncbi.nlm.nih.gov/24874320/
- Gromova OA et al (2015). Neurotrophic and antioxidant potential of neuropeptides and trace elements. https://www.researchgate.net/publication/307647263_Neurotrophic_and_antioxidant_potential_of_neuropeptides_and_trace_elements
- Poon W et al (2015) Cerebrolysin Asian Pacific trial in acute brain injury and neurorecovery: design and methods. https://pubmed.ncbi.nlm.nih.gov/25222349/
- Serge Gauthier et al (2015). Cerebrolysin in mild-to-moderate Alzheimer’s disease: a meta-analysis of randomized controlled clinical trials. https://pubmed.ncbi.nlm.nih.gov/25832905/
- Won Hyuk Chang et al (2016). Cerebrolysin combined with rehabilitation promotes motor recovery in patients with severe motor impairment after stroke. https://bmcneurol.biomedcentral.com/articles/10.1186/s12883-016-0553-z
- Guekht A et al (2017). Safety and efficacy of Cerebrolysin in motor function recovery after stroke: a meta-analysis of the CARS trials. https://pubmed.ncbi.nlm.nih.gov/28707130/
- Robert Teasell et al (2018). Evidence-Based Review of Stroke Rehabilitation. https://www.ebrsr.com/sites/default/files/Ch.%2010%20Upper%20Extremity%20Motor%20Interventions_v20.pdf
- Zavadenko NN et al (2018). Human prion diseases: current issues. https://www.mediasphera.ru/issues/zhurnal-nevrologii-i-psikhiatrii-im-s-s-korsakova/2018/6/downloads/ru/1199772982018061088
- Shuhui Cui et al (2019). Cerebrolysin for vascular dementia. https://pubmed.ncbi.nlm.nih.gov/31710397/
- Torshin I Yu et al (2019). Cerebrolysin peptides as mood stabilizers. https://pubmed.ncbi.nlm.nih.gov/31994517/
- Ivanova NE et al (2020). Review of results from the CAPTAIN II trial: efficacy and safety of Сerebrolysin in neurorecovery after moderate-severe traumatic brain injury. https://cerebrolysin.com.ua/fileadmin/user_upload/TBI/CAPTAIN-Material.pdf
- Muresanu DF et al (2020). Efficacy and safety of cerebrolysin in neurorecovery after moderate-severe traumatic brain injury: results from the CAPTAIN II trial. https://pubmed.ncbi.nlm.nih.gov/31897941/
- Gavrilova SI, Alvarez A (2020). Cerebrolysin in the therapy of mild cognitive impairment and dementia due to Alzheimer’s disease: 30 years of clinical use. https://pubmed.ncbi.nlm.nih.gov/32808294/
- Sherifa AH (2021). Cerebrolycin for Treatment of Covid-related Anosmia and Ageusia. https://clinicaltrials.gov/ct2/show/NCT04830943
- Quinn TJ et al (2021). European Stroke Organisation and European Academy of Neurology joint guidelines on post-stroke cognitive impairment. https://onlinelibrary.wiley.com/doi/10.1111/ene.15068






For IM injections, can I do 10 separate 5mL injections a day in order to get in 50mL?
Hi Sol,
Official instruction recommends to do it through slow intravenous infusions after dilution with the proposed standard solutions for infusion. However, I believe you can do several injections. Be sure to change the place of the injection from time to time.
What would implications be if it was all injected fast within 10-15 seconds? Thanks
Hi Bryan,
It is definitely nothing fatal but should be rather painful. It should be extremely unpleasant due to large volume of liquid being injected. Probably you will be feeling burning sensation in the place of injection, it may also cause bruises. As for the Cerebrolysin effects, they should not be affected. Nonetheless I would recommend to follow the instruction. If you don’t want to do the infusion, you can do several injections in different places.
Any info on quad injections? Would that be suitable considering there might be a localized effect according to Leo? Thanks again
Would a quad injection be suitable? According to Leo there is a localized effect? Thanks again
Hi Bryan!
We have also watched the Leo’s amazing video. Unfortunately, the sources that we have excess to do not state anything about the localized effect. ♂️
so, a quad injection is suitable?…injecting it straight into the blood stream like you inject meth,coke,heroin is not suitable? how much is 10ml? i have used 1cc syringes before. is 10 ml significantly more than that?
Hi Fon, if by quad injection you mean injecting by using four syringes, yes, you can do that if you want.
No, it is not like with those substances you’ve mentioned. As far as I am aware those are usually injected in your vein. Cerebrolysin, on the other hand, is injected into muscle tissue, usually in your thighs. But other muscles are just as fine. However, you never should inject cerebrolysin intervenously. This can be dangerous for your health.
1ml = 1cc, so yes, it is 10 times as large. 10 ml should be injected into a muscle slowly, over one-two minutes.
Hi, I used cerabroylsin injectable 200mg/ml a while ago. I have the old ampule, it says “10ml” , it was from a compounding pharmacy. I recall I used insulin needles into my thigh. naturally I’d like to order the same product. is your 10ML product 10ML per ampule or 10ML per injection ? Thank you
Hi,
We have 10 ml ampules here https://cosmicnootropic.com/products/cerebrolysin. As for the volume of the injections, it depends. If you are using it as a general nootropic, your dosage will be 2-5 ml. As you said, it can be injected with insulin needles. So if you are ordering 10ml ampules, you can keep opened ampule in a fridge for a day or two between the injections.
Is it possible to do the injection subcutaneously? 2-5 mL? Or is this not advised?
Hi Rahul! It is not recommended to inject Cerebrolysin subcutaneously. The preparation should be injected intramuscularly or intravenously.
Related articles
DIY Crypto Wallet: A Step-by-Step Guide
Discover what a crypto wallet is, how it functions, and its significance in storing, sending, and receiving cryptocurrencies.
Thymus Peptides: Innovative Allies in Immune System Enhancement and Cancer Therapy
Learn what are Thymus peptides and about their claimed ability to slow down aging and initiate the body’s recovery processes.
How Long Does it Take for Noopept to Kick In?
Noopept is a synthetic pharmaceutical, categorized as a nootropic (smart…
Can Noopept Cause Depression?
Noopept is known for its cognitive-enhancing and neuroprotective characteristics. It’s…
Thank you!
You will now receive regular updates from us!
Your coupon