Picamilon Injection Instruction
International Non-Proprietary Name (INN): nicotinoyl-gamma-aminobutyric acid
Dosage form: solution for intravenous and intramuscular administration. One ampoule contains 2ml of the substance.
Structure:
Active ingredient: Nicotinoyl gamma-aminobutyrate sodium – 50 mg and 100 mg;
Excipients: hydrochloric acid solution 1 M* – to pH from 6.7 to 8.0; water for injection – up to 1 ml. * – Hydrochloric acid solution 1 M is obtained from concentrated hydrochloric acid and water for injection.
Description
Transparent colorless or slightly brownish liquid.
Pharmacological classification: nootropic
ATC code: N06BX

Table of Contents
- Pharmacodynamics
- Pharmacokinetics
- Intended Uses
- Contraindications
- Pregnancy & Breastfeeding
- Dosage & Administration
- Side Effects, Overdose
- Interaction with Other Drugs
- Special instructions
- Storage Conditions & Shelf life
Pharmacodynamics:
Nootropic drug that dilates cerebral vessels. It has tranquilizing and mild psychostimulant effect, antiaggregant and antioxidant effect. Improves the functional state of the brain by normalizing tissue metabolism and influence on cerebral blood circulation (increases the volume and linear velocity of cerebral blood flow, has a vasodilatory, antiaggregant, antioxidant effect, improves microcirculation). With course treatment increases physical and mental performance, reduces headache, improves memory, normalizes sleep, helps reduce or eliminate feelings of anxiety, tension, fear, emotional intemperance. Contributes to the restoration of central motor and speech functions in case of disorders of vascular or traumatic genesis. Improves blood circulation in the vessels of the retina and optic nerve.
Pharmacokinetics:
Absorption is rapid and complete by any routes of administration. The drug penetrates through the blood-brain barrier, and is retained in body tissues for a long time. Bioavailability – 50-88%. Excreted mainly by the kidneys in unchanged form. The elimination half-life is 0.51 hours.
Indications for Use:
Adults:
- chronic cerebrovascular insufficiency (dyscirculatory encephalopathy, consequences of cerebral circulatory disorders), conditions after traumatic brain injury, vegetative dystonia syndrome, as part of the complex therapy of depressive disorders of various genesis in old age
- as part of the complex treatment of chronic alcoholism (reduction of asthenic, asthenoneurotic, post-psychotic, pre-relapsing states in alcoholic encephalopathy).
- as part of the complex therapy of primary open-angle glaucoma with compensated intraocular pressure (to stabilize visual function).
- as part of the complex therapy of vegetative dystonia syndrome, accompanied by anxiety, fear, increased irritability, asthenia, emotional lability.
- to improve tolerance to physical and mental stress, increase stress resistance (for people in stressful and extreme conditions; to restore physical performance of athletes, to increase resistance to physical and mental stress).
Children over 3 years old and adults in urological practice for urinary disorders to improve the adaptation function of the bladder (reduction of detrusor hypoxia).
Contraindications: Hypersensitivity to the components of the drug, chronic renal failure, pregnancy, breastfeeding, children under 3 y.o. (in children over 3 y.o. the drug is used for urinary disorders).
Use in pregnancy and during breastfeeding: Due to the fact that the safety of the drug use in pregnant and lactating women has not been established, the use of the drug during pregnancy and breastfeeding is contraindicated.
Administration and Dosage:
Intravenously by drip or bolus (slowly); intramuscularly. Before drip intravenous administration the contents of the ampoule are dissolved in 200 ml of 0.9% sodium chloride solution.
- In chronic cerebrovascular insufficiency: 100-200 mg once a day for 10 days intravenously, then intramuscularly. Course of treatment 15 – 30 days. With improvement switch to oral forms in a daily dose of 50-150 mg. The repeated course – after 5-6 months.
- As part of complex therapy of depressive states of the elderly: 50-200 mg per day for 10-15 days.
- In the treatment of chronic alcoholism: 100 mg per day for 6-15 days.
- To restore performance under increased loads: 200 mg once a day for 10-15 days, for athletes – the same dose for 14 days of training period.
- As part of the complex therapy of vegetative dystonia syndrome: 100-200 mg for 10-15 days.
- In the complex treatment of primary open-angle glaucoma: 100-200 mg per day for 10 days.
- In the treatment of urinary disorders: 100 mg intramuscularly (for children from 3 to 10 years old), 200 mg per day (for children over 11 y.o. and adults).
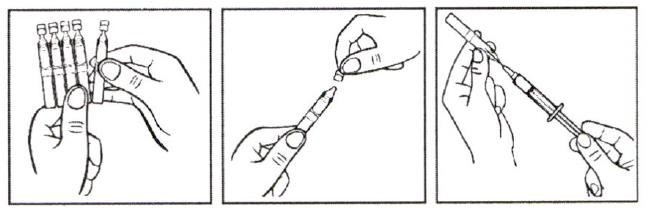
How to use a polymer ampoule:
- Take the ampoule and shake it by holding its neck
- Rotate and separate the valve
- Use a needle to draw up the contents of the ampoule into the syringe
Side Effects: Decreased blood pressure, dizziness, headache, mild nausea, irritability, agitation, anxiety, allergic reactions (skin rash, itching).
Overdose: Increased severity of dose-dependent symptoms of adverse effects. Treatment is symptomatic.
Drug Interaction: Picamilon shortens the effect of barbiturates, enhances the effect of narcotic analgesics.
Influence on the ability to drive vehicles and operate machines: During the treatment, caution shall be taken when driving vehicles and engaging in other potentially hazardous activities requiring increased concentration and quick psychomotor reactions since the drug can have a negative effect on the speed of reaction.
Storage: Store at room temperature not exceeding 25°C (77°F) in original packaging. Keep in a safe place out of reach of children.
Shelf life: 2 years. Do not use after the expiration date printed on the package.
Manufacturer: PharmStandart, www.pharmstd.com